Our bodies are extraordinarily complex active machines; myriads of pumps, plumbing, filters, and scaffolds which are not only undergoing continuous repair, but adapting in response to mechanical forces. Force-sensing and mechanical adaptation can be seen in nearly every aspect of our physiology[1,2]; for example, vascular cells undergo profound morphological changes in response to blood flow shear stresses; these responses have implications in the physiological function of blood vessels, particularly in disorders such as in atherosclerosis and thrombosis[3,4]. Force-mediated changes in cell mechanics also are critical in pathological states; in kidney health, the filtration barrier appears to be regulated by mechanosensing, and genetic defects in this sensing lead to proteinuria and renal failure[5]. In cancer, a disease characterized by diverse biochemical changes, cells appear to universally soften and generate higher forces[9,10], potentially allowing them to invade new tissue more readily. Additionally, metastatic cells’ mode of movement may switch from crawling to "blebbing", where they move ~20x faster through fibrous tissues, presenting a far more invasive and difficult cancer[6,7].
As such, physical mechanics has now become widely accepted as critically regulating biology[8]. The internal mechanics of biology are largely set by a collection of intracellular biopolymers known as the cytoskeleton, the cellular scaffold essential for virtually every aspect of life, and its disruption is the root cause of many aforementioned diseases[9,10]. Similarly, the therapeutic mechanism of actions for several drugs directly or indirectly affect this cellular scaffold[9], making it important to understand the changes they impose on the mechanical state of the cell.
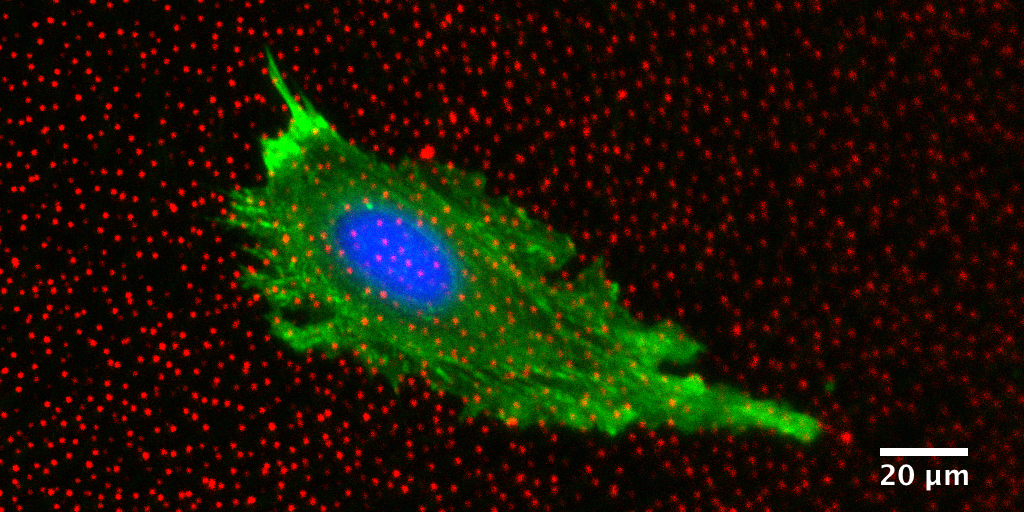
Quantitative morphological and mechanical characterization of cells can therefore be an important measure of their degree of deviation from a healthy state, or alternately of their responsiveness to a drug[9]. One such method is Traction Force Microscopy (TFM), which quantifies the forces that cells exert on their surrounding environment[9]. These methods could be used as new metrics for cell response to drugs in development and would offer a unique perspective on physiology.
References
- Vogel, V. & Sheetz, M. Local force and geometry sensing regulate cell functions. Nat Rev Mol Cell Biol 7, 265-275 (2006).
- Wang, N., Tytell, J. D. & Ingber, D. E. Mechanotransduction at a distance: mechanically coupling the extracellular matrix with the nucleus. Nat Rev Mol Cell Biol 10, 75-82, doi:nrm2594 [pii] 10.1038/nrm2594 (2009).
- Davies, P. F., Spaan, J. A. & Krams, R. Shear stress biology of the endothelium. Ann Biomed Eng 33, 1714-1718 (2005).
- Hahn, C. & Schwartz, M. A. Mechanotransduction in vascular physiology and atherogenesis. Nat Rev Mol Cell Biol 10, 53-62, doi:nrm2596 [pii] 10.1038/nrm2596 (2009).
- Akilesh, S. et al. Arhgap24 inactivates Rac1 in mouse podocytes, and a mutant form is associated with familial focal segmental glomerulosclerosis. J Clin Invest 121, 4127-4137, doi:10.1172/JCI46458 (2011).
- Sanz-Moreno, V. et al. Rac activation and inactivation control plasticity of tumor cell movement. Cell 135, 510-523, doi:S0092-8674(08)01236-1 [pii] 10.1016/j.cell.2008.09.043 (2008).
- Saito, K., Ozawa, Y., Hibino, K. & Ohta, Y. FilGAP, a Rho/ROCK-regulated GAP for Rac controls tumor cell migration. Mol Biol Cell, doi:mbc.E12-04-0310 [pii] 10.1091/mbc.E12-04-0310 (2012).
- Ehrlicher, A. J., Nakamura, F., Hartwig, J. H., Weitz, D. A. & Stossel, T. P. Mechanical strain in actin networks regulates FilGAP and integrin binding to filamin A. Nature (2011).
- Yoshie H, Koushki N, Kaviani R, Tabatabaei SM, Rajendran K, Dang Q, Husain A, Yao S, Li C, Sullivan J, Saint-Geniez M, Krishnan R*, Ehrlicher AJ*. Traction force screening enabled by compliant PDMS elastomers. Biophys J, 114(9): 2194-2199, 2018. * contributed equally
- Yoshie H, Koushki N, Molter C, Siegel PM, Krishnan R, Ehrlicher AJ. High Throughput Traction Force Microscopy Using PDMS Reveals Dose-Dependent Effects of Transforming Growth Factor-β on the Epithelial-to-Mesenchymal Transition. J Vis Exp. Jun 1;(148), 2019.